DL-Sulforaphane Powder
Bulk Sulforaphane Extract Manufacturer & Supplier | GMP Factory in China
DL-Sulforaphane (CAS 4478-93-7) is a high-value bioactive isothiocyanate ingredient derived from cruciferous plant sources, widely researched and utilized in nutraceutical, functional food, cosmetic, and scientific formulation applications.
AIHerba® (Shaanxi Zhonghong Investment Technology Co., Ltd.) is a GMP-certified DL-Sulforaphane manufacturer and bulk supplier, offering standardized, traceable, and specification-customizable sulforaphane powder for global B2B buyers. With over 28 years of plant extract manufacturing experience, advanced analytical systems (HPLC, ICP-MS, GC-MS), and strict quality control protocols, we provide stable batch-to-batch consistency, full COA documentation, and OEM/ODM support.
Our DL-Sulforaphane is suitable for nutraceutical formulations, functional ingredient systems, cosmetic antioxidant complexes, and research applications, meeting international quality, safety, and regulatory expectations for professional buyers.
👉 Request samples, COA, or bulk quotations directly from our factory.
1. What Is DL-Sulforaphane?
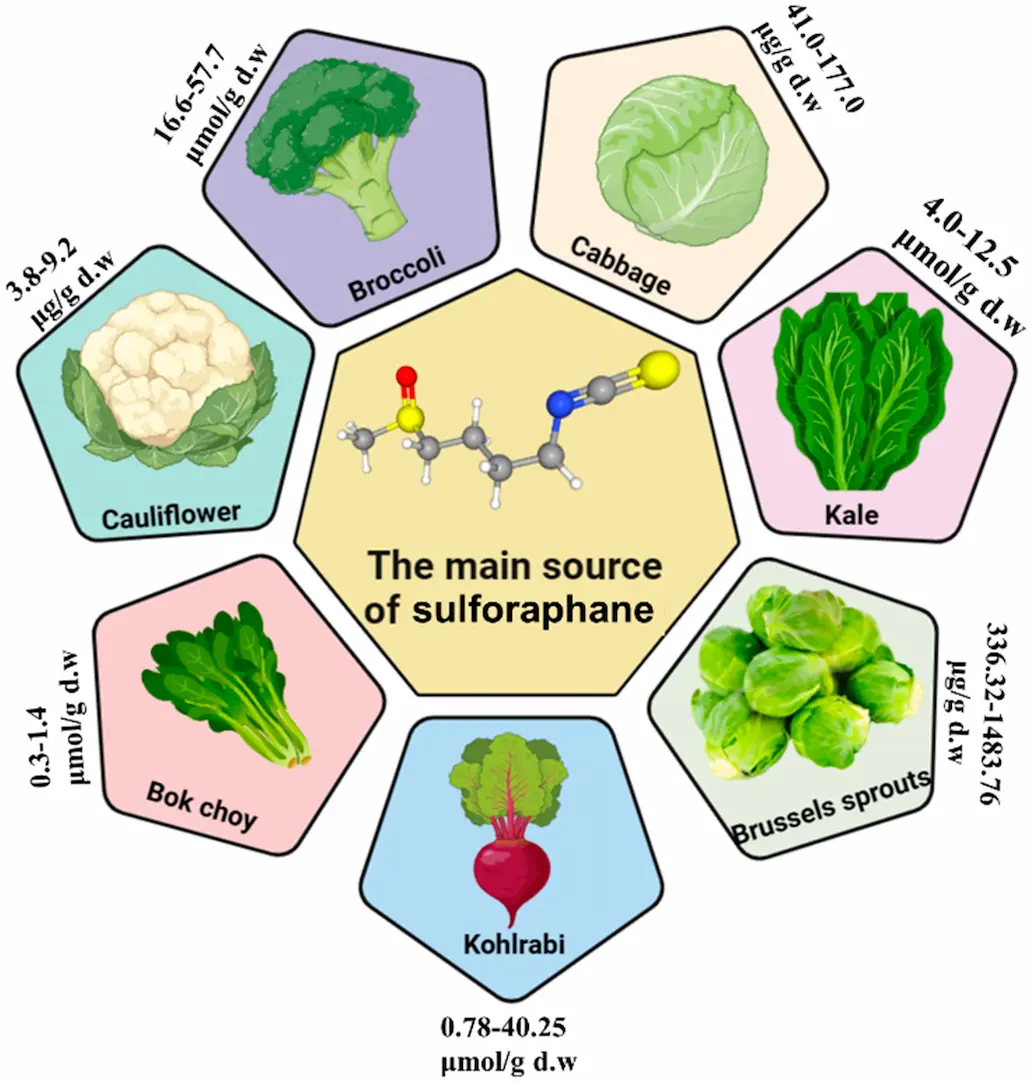
DL-Sulforaphane is a naturally derived isothiocyanate compound formed through enzymatic conversion of glucoraphanin, a sulfur-containing precursor found in broccoli sprouts and other cruciferous vegetables.
In industrial and research contexts, DL-Sulforaphane is valued as a functional bioactive ingredient due to its well-studied role in cellular antioxidant signaling pathways and its compatibility with modern nutraceutical and cosmetic formulations.
Important Notice:
DL-Sulforaphane is supplied as a raw material ingredient. Information provided here is for scientific, technical, and formulation reference only and does not constitute medical advice or therapeutic claims.
2. Product Source & Raw Material Traceability
-
Botanical Source: Broccoli sprouts / cruciferous plant materials
-
Cultivation: Contract-farmed, pesticide-controlled sourcing
-
Harvest Stage: Optimized for glucoraphanin concentration
-
Traceability: Full batch traceability from raw material to finished powder
AIHerba maintains long-term agricultural sourcing partnerships to ensure consistency, sustainability, and regulatory compliance.
3. Technical Specifications (Key Parameters)
| Item | Specification |
|---|---|
| Product Name | DL-Sulforaphane |
| CAS Number | 4478-93-7 |
| Molecular Formula | C₆H₁₁NOS₂ |
| Appearance | White to off-white powder |
| Odor | Characteristic |
| Purity | Customizable (≥1%, ≥10%, ≥98%) |
| Test Method | HPLC |
| Solubility | Water / ethanol-compatible |
| Shelf Life | 24 months (recommended storage) |
4. Product Specifications & COA
4.1 Pesticide Residues
| Category | Item | Limit | Detection Method |
|---|---|---|---|
| Pesticides | Chlorpyrifos | ≤ 0.01 ppm | GC-MS |
| Pesticides | Cypermethrin | ≤ 0.02 ppm | GC-MS |
| Pesticides | Carbendazim | ≤ 0.05 ppm | HPLC-MS/MS |
4.2 Heavy Metals
| Category | Item | Limit | Detection Method |
|---|---|---|---|
| Heavy Metals | Lead (Pb) | ≤ 0.5 ppm | ICP-MS |
| Heavy Metals | Mercury (Hg) | ≤ 0.01 ppm | CVAAS |
| Heavy Metals | Cadmium (Cd) | ≤ 0.05 ppm | ICP-MS |
4.3 Microbiological Parameters
| Category | Item | Limit | Detection Method |
|---|---|---|---|
| Microbial | Total Plate Count | ≤ 100 CFU/g | Plate Count |
| Pathogens | E. coli | Absent | PCR / Plating |
| Pathogens | Salmonella | Absent | PCR / Plating |
| Pathogens | Listeria monocytogenes | Absent | PCR / Plating |
| Pathogens | Vibrio spp. | Absent | PCR / Plating |
✔ Full COA, MSDS, and batch reports available upon request.
5. Production Process Overview
-
Raw Material Selection & Inspection
-
Enzymatic Conversion
Controlled hydrolysis converts glucoraphanin into sulforaphane under low-temperature conditions. -
Solvent Extraction
Food-grade water-ethanol systems ensure safety and efficiency. -
Purification & Concentration
Column chromatography and preparative HPLC for impurity removal. -
Drying & Stabilization
Vacuum freeze-drying or spray drying. -
Packaging & Batch Coding
Light-resistant, sealed packaging with full traceability.
6. Mechanism of Action (Scientific Context)
From a biochemical perspective, sulforaphane has been extensively studied for its interaction with cellular antioxidant response elements, particularly its role in activating Nrf2-related signaling pathways under laboratory conditions.
These mechanisms are primarily explored in in vitro and preclinical research settings and serve as the scientific basis for its application as a functional ingredient in formulation science.
7. Usage Guidelines & Formulation Reference
For formulation and industrial reference only
-
Nutraceutical formulations: Typically used as a functional active ingredient within standardized formulations.
-
Cosmetic systems: Incorporated into antioxidant or protective complexes.
-
R&D applications: Applied according to experimental design protocols.
⚠️ Final dosage, claims, and labeling must comply with local regulatory frameworks and are the responsibility of the finished-product manufacturer.
8. Traditional & Historical Context (Non-Medical)
Cruciferous vegetables have a long history of dietary use across various cultures. Modern extraction technologies allow specific bioactive components, such as sulforaphane, to be studied and applied as isolated functional ingredients in contemporary product development.
9. Storage, Shelf Life & Packaging
-
Storage: Cool, dry, light-protected environment
-
Shelf Life: 24 months
-
Packaging Options:
-
1 kg aluminum foil bags
-
5–25 kg fiber drums
-
Custom OEM packaging available
-
10. Samples, MOQ & Logistics
-
Sample Policy: Available upon request
-
MOQ: Negotiable (based on specification and customization)
-
Shipping: DHL / FedEx (samples), Air or Sea freight (bulk)
-
Global Export Experience: 30+ countries
11. Industry Applications & Real-World Scenarios
Scenario 1: Nutraceutical Manufacturer
Used as a functional antioxidant ingredient in capsule or powder formulations targeting premium supplement markets.
Scenario 2: Cosmetic Raw Material Supplier
Integrated into anti-oxidative skincare ingredient systems for stability-focused formulations.
Scenario 3: Research & Development Institutions
Applied as a standardized reference compound for laboratory studies and formulation testing.
12. Comparative Purchasing Guide
| Factor | AIHerba® |
|---|---|
| Manufacturing Experience | 28+ years |
| Certifications | GMP, ISO |
| Analytical Capability | HPLC, ICP-MS, GC-MS |
| Custom Specifications | ✔ |
| OEM / ODM Support | ✔ |
| Traceable Supply Chain | ✔ |
13. Certifications & Authority
-
GMP Certified Manufacturing Facility
-
ISO 9001 Quality Management
-
Internal QA/QC Laboratory
-
Full Documentation Support
14. Frequently Asked Questions (FAQ)
-
Is DL-Sulforaphane suitable for nutraceutical manufacturing?
Yes, it is commonly used as a functional ingredient subject to local regulations. -
Do you provide COA and MSDS?
Yes, full documentation is available. -
Can purity specifications be customized?
Yes, we support customized purity levels. -
Is OEM service available?
Yes, OEM & ODM solutions are supported. -
What test methods are used?
HPLC, ICP-MS, GC-MS, microbiological testing. -
Do you ship globally?
Yes, we export worldwide.
15. References & Regulatory Context
-
National Institutes of Health (NIH): Sulforaphane Research
https://pubmed.ncbi.nlm.nih.gov -
FDA Dietary Ingredient Guidance
https://www.fda.gov -
EFSA Botanical Ingredient Safety Framework
https://www.efsa.europa.eu
About AIHerba®
AIHerba® | China Natural Plant Extracts Supplier & OEM Factory
Shaanxi Zhonghong Investment Technology Co., Ltd. is a GMP-certified manufacturer specializing in high-purity natural plant extracts for nutraceutical, cosmetic, functional food, and pharmaceutical industries.
📧 sales@aiherba.com | info@aiherba.com | liaodaohai@gmail.com
🌐 https://www.aiherba.com





