1. Ashwagandha Extract (Withania somnifera) – Bulk Herbal Extract Supplier
Ashwagandha Extract (Withania somnifera) is a high-quality herbal raw material sourced directly from certified farms and processed under ISO, HACCP, and GMP standards. Our extract is available in standardized concentrations, meeting rigorous quality controls with COA and third-party testing verification (HPLC, UV, microbial). Trusted by international supplement, functional food, and cosmetic manufacturers, our Ashwagandha extract is produced in our vertically integrated factory, ensuring traceability from farm to final product. Bulk supply, custom specifications, and professional support are available to meet B2B procurement needs. With detailed specifications, safe handling guidelines, and industrial applications, our Ashwagandha extract page provides a complete resource for professional buyers seeking reliable and compliant herbal raw materials.
2. Product Overview
-
Scientific Name: Withania somnifera
-
English Name: Ashwagandha Extract
-
Form: Powder / Fine Powder / Water-Soluble / Oil-Soluble
-
Appearance: Yellowish-brown fine powder
-
CAS No.: 90147-43-6
-
Plant Part Used: Root / Whole Plant
-
Lead Time: 3–7 Working Days
-
Packing: 25 kg/drum; 27 drums/tray
-
Main Markets: Europe, North America, Asia, Middle East
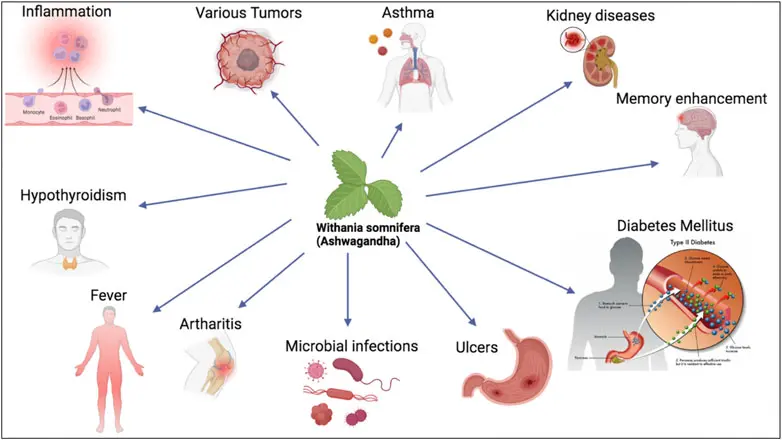
3. Benefits & Functional Profile (Scientific & Neutral Language)
-
Rich in bioactive compounds such as Withanolides, providing standardization for industrial use.
-
Used as a raw material for functional foods, dietary supplements, cosmetics, and herbal formulations.
-
Recognized in traditional herbal systems for adaptogenic properties (non-medical description, reference to traditional use only).
-
Processed under controlled conditions to maintain phytochemical integrity.
Ashwagandha Benefits
4. Usage Guidelines & Traditional Applications
-
Usage Recommendations: Follow recommended incorporation rates in finished products. For cosmetic formulations, add during the appropriate phase to ensure stability.
-
Traditional Applications: Commonly used in herbal formulations, wellness powders, teas, and functional foods.
-
Recommended Dosage (Industrial Use): 2–5% for powder blends; 0.5–2% for cosmetic formulations (adjust according to formulation requirements).
-
Optimal Formulation Advice: Compatible with herbal blends, plant extracts, and liquid carriers. Stability tested under standard processing conditions.
5. Product Specifications & Certificates of Analysis (COA)
| Category | Parameter / Name | Specification / Limit | Testing Method |
|---|---|---|---|
| Ashwagandha Extract | Withanolides (HPLC) | ≥5% | HPLC |
| Ashwagandha Extract | Total Ash (%) | ≤5% | AOAC |
| Moisture (%) | ≤8% | Karl Fischer |
Pesticide Residues
| Residue Item | Limit | Testing Method |
|---|---|---|
| Organophosphates | ND | GC-MS |
| Chlorpyrifos | ND | GC-MS |
| Others | ND | GC-MS |
Heavy Metals
| Metal | Limit | Testing Method |
|---|---|---|
| Lead (Pb) | ≤2 ppm | ICP-MS |
| Arsenic (As) | ≤1 ppm | ICP-MS |
| Mercury (Hg) | ≤0.1 ppm | ICP-MS |
| Cadmium (Cd) | ≤1 ppm | ICP-MS |
Microbial Limits
| Microbial Item | Limit | Testing Method |
|---|---|---|
| Total Plate Count | ≤1000 CFU/g | AOAC |
| Yeast & Mold | ≤100 CFU/g | AOAC |
| E. coli | Negative | AOAC |
| Salmonella | Negative | AOAC |
6. Production & Manufacturing Process
-
Raw Material Traceability: Sourced from certified farms with documented origin.
-
Processing: Extraction via ethanol/water-based methods under controlled temperatures to preserve bioactive compounds.
-
Filtration & Concentration: Ensures uniform quality and standardized extract levels.
-
Drying & Packaging: Low-temperature spray drying, packed under nitrogen to prevent oxidation.
-
Production under ISO / HACCP / GMP: Full compliance with international standards.
-
Third-Party Testing: HPLC for active compounds, UV spectroscopy, microbial analysis.
7. Storage & Shelf Life
-
Storage: Keep in a cool, dry place, away from direct sunlight and moisture.
-
Shelf Life: 24 months when stored under recommended conditions.
-
Packaging: 25 kg/drum, 27 drums per pallet; customized packaging available.
8. Sample Policy, Transport & MOQ
-
Samples: Available for evaluation prior to bulk order.
-
Transportation: Air / Sea shipping options, temperature-controlled options for sensitive batches.
-
Minimum Order Quantity (MOQ): 25 kg per order; bulk pricing available.
9. Applicable Industries & Application Scenarios
-
Industries: Dietary supplements, functional food, herbal products, cosmetics, nutraceuticals, pharmaceutical raw material.
-
Application Scenarios: Capsules, tablets, powders, beverages, creams, and serums.
-
Usage Method: Incorporate according to formulation requirements; ensure compatibility with other ingredients.
10. Scientific Source & Quality Assurance
-
Raw Material Traceability: Farm-to-factory documentation, COA available.
-
Production under ISO / HACCP / GMP: Certified processing facility.
-
Third-Party Testing: HPLC, UV, Microbial testing ensure product compliance.
-
References & Literature:
-
PubMed: Withania somnifera review
-
11. FAQ (Frequently Asked Questions)
-
Is this Ashwagandha extract suitable for vegan products?
Yes, plant-based and free from animal-derived components. -
Do you provide Certificate of Analysis (COA)?
Yes, each batch comes with COA and third-party testing report. -
Can I request custom standardization?
Yes, we provide flexible specifications upon request. -
What is the recommended shelf life?
24 months when stored properly in sealed containers. -
How can I order samples?
Contact sales@aiherba.com for sample requests.
12. Factory Profile & Brand Assurance
-
Experience: Over 15 years of herbal extract production with professional R&D team.
-
Certifications: ISO 9001, HACCP, GMP, Organic Certified (where applicable).
-
Quality Control: Complete traceability, standardized testing, in-house QA/QC team.
-
Customer Cases: Supplied to international supplement and cosmetic brands with verified quality.
-
Contact: sales@aiherba.com | +86-19929018987
13. References
-
PubMed: https://pubmed.ncbi.nlm.nih.gov/
-
FDA Dietary Supplement Guidelines: https://www.fda.gov/
-
NIH Herbal Database: https://www.nih.gov/
-
AOAC Official Methods: https://www.aoac.org/
Bulk Supply & Technical Support
Get direct factory quotes, COA, and MSDS within 12 hours. We support bulk supply and custom specifications.