1. Melatonin API: Benefits, Specifications, COA & Uses
Melatonin is a biologically active indoleamine widely used as a functional ingredient in dietary supplements and sleep-support formulations. AIHerba® supplies high-purity Melatonin API (≥99%, HPLC-verified) manufactured under GMP-compliant conditions, designed specifically for bulk procurement, OEM formulation, and global regulatory alignment.
Unlike consumer-facing melatonin products, this ingredient-grade melatonin is intended for professional manufacturers, brand owners, and contract formulators requiring precise dosage control, batch-to-batch consistency, and full analytical documentation. Each batch is supported by COA, impurity profiling, and stability data, enabling smooth product registration and quality audits in international markets.
With over 28 years of experience in nutraceutical ingredient manufacturing, AIHerba® provides scalable supply, technical support, and traceable production for melatonin-based formulations across dietary supplements, functional foods, and customized OEM solutions.
2. What Is Melatonin? (Ingredient-Level Definition)
Melatonin (CAS No. 73-31-4) is an endogenous hormone naturally produced by the pineal gland in humans. From an industrial perspective, melatonin used in supplements is chemically synthesized to achieve pharmaceutical-grade purity, stability, and dosage accuracy.
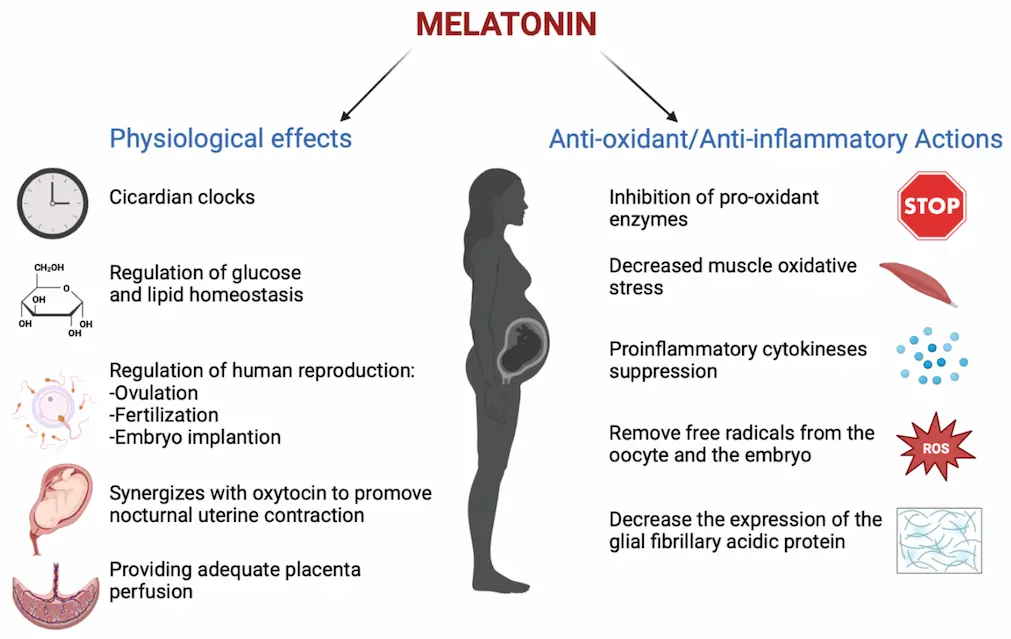
As an ingredient, melatonin functions as a chronobiotic compound, supporting circadian rhythm signaling rather than acting as a sedative. This distinction is critical for regulatory compliance and product positioning.
3. Product Source & Manufacturing Origin
-
Ingredient Type: Synthetic Melatonin API
-
Manufacturing Site: GMP-certified facility, China
-
Supplier: AIHerba® (Shaanxi Zhonghong Investment Technology Co., Ltd.)
-
Production Standard: GMP, ISO-compliant quality system
-
Traceability: Full batch records and raw material traceability available
4. Technical Specifications (Key Parameters)
| Parameter | Specification |
|---|---|
| Appearance | White or off-white crystalline powder |
| Assay | ≥99.0% (HPLC) |
| Molecular Formula | C₁₃H₁₆N₂O₂ |
| Molecular Weight | 232.28 |
| Solubility | Slightly soluble in water, soluble in ethanol |
| Stability | Stable under recommended storage conditions |
5. Mechanism of Action (Non-Therapeutic)
From a biochemical perspective, melatonin interacts with MT1 and MT2 receptors involved in circadian rhythm signaling. It does not induce sleep directly, but supports physiological time signaling when formulated and dosed appropriately.
This mechanism explains its wide use in sleep-support supplements, jet lag formulations, and circadian rhythm products without making medical claims.
6. Health-Related Benefits (Regulatory-Safe Language)
-
Supports normal circadian rhythm regulation
-
Helps align sleep-wake timing
-
Widely studied for sleep onset support
⚠️ No disease treatment or prevention claims are made.
7. Usage Guidelines (For Formulation Reference Only)
-
Typical formulation range: 0.5–5 mg per serving
-
Formulation types: Capsules, tablets, gummies, powders
-
Compatibility: Suitable for single-ingredient or blended formulas
Final dosage must comply with local regulatory limits.
8. Traditional & Historical Context (Brief)
Melatonin’s discovery in the mid-20th century led to extensive research into biological rhythms. While not a traditional herbal ingredient, it has become one of the most researched chronobiotic compounds globally.
9. Recommended Formulation Strategies
-
Low-dose precision formulations
-
Combination with magnesium, L-theanine, or vitamin B6
-
Time-release or microencapsulation for stability
10. Safety Notes & Precautions
-
Not intended for pharmaceutical use
-
Not recommended for pregnant or breastfeeding populations
-
Potential interactions with anticoagulants and immunosuppressants
11. COA & Quality Control Parameters
Pesticide Residues
| Item | Limit | Method |
|---|---|---|
| Organophosphates | Conform USP | GC-MS |
| Organochlorines | Conform USP | GC-MS |
| Pyrethroids | Conform USP | GC-MS |
Heavy Metals
| Item | Limit | Method |
|---|---|---|
| Lead (Pb) | ≤1.0 ppm | ICP-MS |
| Arsenic (As) | ≤1.0 ppm | ICP-MS |
| Cadmium (Cd) | ≤0.5 ppm | ICP-MS |
| Mercury (Hg) | ≤0.1 ppm | ICP-MS |
Microbiological Limits
| Item | Limit | Method |
|---|---|---|
| Total Plate Count | ≤1,000 CFU/g | USP <61> |
| Yeast & Mold | ≤100 CFU/g | USP <61> |
| E. coli | Negative | USP <62> |
| Salmonella | Negative | USP <62> |
12. Production Process Overview
-
Raw material synthesis
-
Crystallization & purification
-
HPLC assay verification
-
Impurity & residual solvent testing
-
Packaging under controlled environment
13. Storage, Shelf Life & Packaging
-
Storage: Cool, dry, light-protected
-
Shelf Life: 24 months
-
Packaging: 1 kg / 5 kg / 25 kg aluminum foil bags or fiber drums
14. Sample Policy, Shipping & MOQ
-
Samples: Available upon request
-
MOQ: 1 kg (R&D), 25 kg (commercial)
-
Shipping: Air / Sea / Express (DHL, FedEx)
15. Clinical Data & Research Overview
-
NIH NCCIH: Overview of melatonin research
-
PubMed meta-analyses on sleep onset latency
-
EFSA scientific opinions on melatonin claims
16. Applicable Industries & Use Cases
Case 1: Dietary Supplement Brands
Capsules, tablets, gummies requiring precise low-dose control.
Case 2: OEM/ODM Manufacturers
Private label sleep-support formulations.
Case 3: Functional Nutrition R&D
Combination formulas targeting circadian rhythm support.
17. Comparison & Procurement Guide
| Factor | Low-Grade Supplier | AIHerba® |
|---|---|---|
| Assay Accuracy | Variable | ≥99% HPLC |
| Documentation | Limited | Full COA |
| GMP Compliance | Unclear | Verified |
| Technical Support | Minimal | Dedicated |
18. FAQ (6 Questions)
-
Is melatonin pharmaceutical grade?
-
Can you provide batch-specific COA?
-
What markets does this ingredient comply with?
-
Is OEM formulation support available?
-
What is the typical lead time?
-
Can small trial orders be supported?
19. References & Regulatory Sources
-
NIH NCCIH – Melatonin
https://www.nccih.nih.gov/health/melatonin-what-you-need-to-know -
PubMed – Melatonin Research
https://pubmed.ncbi.nlm.nih.gov -
FDA – Dietary Supplement Regulations
https://www.fda.gov/food/dietary-supplements -
EFSA – Melatonin Opinions
https://www.efsa.europa.eu
20. Manufacturer Information
AIHerba® | China Natural Plant Extracts Supplier & OEM Factory
Shaanxi Zhonghong Investment Technology Co., Ltd.
📧 sales@aiherba.com | info@aiherba.com | liaodaohai@gmail.com
🌐 www.aiherba.com





